Give the Products of the Following Acid-base Reactions
Give the products of the following acidbase reactions and indicate whether reactants or products are favored at equilibrium. HCO 3- NH 3----- NH 4 CO 3 2 - CaO H 2 O ----- Ca 2 2 OH -2 H 3 O CO 3 2 -.
Therefore D is correct option.

. CH3CH2OH NH2 c. Indicate which side of the reaction is favored. NH4 s2- s H2SO4 Conjugate acid-base pairs.
Draw the molecules on the canvas by choosing buttons from the Tools. Place the following compounds in order of decreasing acidity. H 2 O NH 3 OH NH 4.
Give the products of the following acid-base reactions. 8 pts HO 0-H H3C-0 H2C HAC-NH2 0-H H3C NH2 0-H H-CI. Give the products of the following acid-base reactions and indicate whether reactants or products are favored at equilibrium use pKa the values that are given in Section 117.
For each of the acid-base reactions. This is the best answer based on feedback and ratings. So we can also say that the end product of an acid base reaction is a salt.
The hydroxide ion is the conjugate base of water which acts as an acid and the ammonium ion is the conjugate acid of the base ammonia. OH -I-AsO 4 3 â NH 2 â HPO 4 2 â NO 2 â Give the conjugate base of the following. CH3COH CH3NH2 d.
Draw the product from the following reactions. CHCHOH NH c. Give the products of the following acid-base reactions and indicate whether reactants or products are favored at equilibrium use the pKa values that are given in Section 118.
Indicate which side of the reaction is favored by looking up the pkas. In acid-base chemistry a salt is an ionic compound formed from. Show the mechanism using formal arrow pushing.
Give the products of the following acid-base reactions and indicate. Ammonia NH3 usually acts as a base in Bronsted-Lowry acid-base reactions but can also act as an acid. Identify the conjugate acid-base pairs Conjugate acid-base pairs.
Was this answer helpful. Give the products of the following acid-base reactions and indicate whether reactants or products are favored at equilibrium. The equation given for this reaction is.
Give the products of the following acid-base reactions and indicate whether. Use the pK_a values in the table below. ACH3COH CH3O b.
Post the pka so that we can pr. Reactions between Arrhenius acids and bases always produce H2O and an ionic compound. ཀཔ H3C-o N-H H H-CI 1.
HCl aq NaOH aq NaCl aq H 2 O l 2 Although simple the Arrhenius model is not particularly useful when it comes to understanding the reactions considered in organic chemistry. Give the products in the following acid-base reactions. Give the products of the following acid-base reactions and indicate whether reactants or products are favored at equilibrium.
What is the product of the following acid-base reaction. Use the pk values that are given in Section 23 CHNH. Here H 2 O donates a proton to NH 3.
Predict the products of the following acid-base reactions and predict whether the equilibrium lies to the left or to the right of the equation. Identify the acid base conjugate acid and conjugate base for each reaction. Get more out of your subscription Access to over 100 million course-specific study resources.
Up to 256 cash back Give the conjugate acids of the following. In a reaction in which ammonia acts as a Bronsted-Lowry acid what must one of the products be. Acetylene alcohol and carboxylic acid all contains acidic hydrogen and Grignard reagent acting as strong base can take up proton.
Give the products of the following acid-base reaction and indicate whether reactants or products are favored at equilibrium. Use the mathrmp K_mathrma values that are given in Section 23. Express your answer as part of a chemical equation.
CHCOH CH0 b. Ammonium chloride is made up of NH 4 cations from the base NH 3 and Cl anions from the acid HCl. The reaction of an acid with a base to produce a salt and water.
S2- H2SO4 Conjugate acid-base pairs HCIO4 NH3 Conjugate acid-base pairs. The end product found is H 2 O ie. The H from the acid and the hydroxide ion OH from the base combine to yield H2O while the remaining ions combine to form an ionic compound.
Okay this question covers determining the product of an acid base reaction and predicting which way will the equilibrium favor. HCl aq NaOH aq H aq Cl aq Na aq OH aq NaCl aq H 2 O l The above reaction shows how the dissociation of HCl and NaOH takes place. CH3OH NH3 2 CHA - NHZOH CH2OH NH3 CH3NH2 Hzo CH3O NHA CH3OH2 NH2.
Hydrochloric acid reacts with ammonia to form ammonium chloride a salt. Indicate which side of the reaction is favored by looking up the pkas. Give the products of the following acid-base reactions.
Show the mechanism using formal arrow pushing. If a substance with a tendency to grab a proton meets a substance with a tendency to give up a proton a proton-transfer reaction can occur. Show the mechanism using formal arrow pushing.
Identify all of the phases in your answer. CHCHOH HCI. A NH4 B NH4 C NH3 D NH3 E NH2 F.
PH 4 HCO 3 â Identify the acid in each of the following and explain your choice. HCl g H 2 O H aq Cl aq sometimes written as or HCl aq AcidBase Reaction. Water molecule and NaCl which is a salt.
Water can also act as an acid as when it reacts with ammonia. In this case we can go ahead and start off with our acetate and our bas. This kind of reaction can be alternatively viewed as a double-displacement reaction.
HCl aq NH 3 aq NH 4 Cl aq You should notice that in the first two examples the base contained OH ions and therefore the products were a salt and water. Give an approximate pka value for each acid and conjugate acid. For each of the acid-base reactions in Section 23 compare the mathr 0425.
H 2 O HCl ----- H 3 O Cl. View the full answer. Give the products of the following acid-base reactions and indicate whether reactants or products are favored at equilibrium.
Predict the products of the following acid-base.

Strong Acid Strong Base Reactions Video Khan Academy
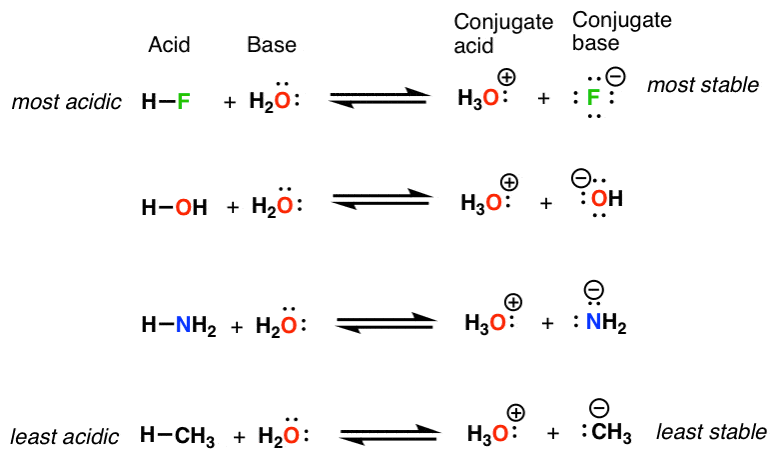
Basicity Is Another Word For Stability Of A Lone Pair Of Electrons

How To Determine The Position Of Equilibrium For An Acid Base Reaction Chemistry Steps

Acid Base Reaction Definition Examples And Uses

How To Determine The Position Of Equilibrium For An Acid Base Reaction Chemistry Steps
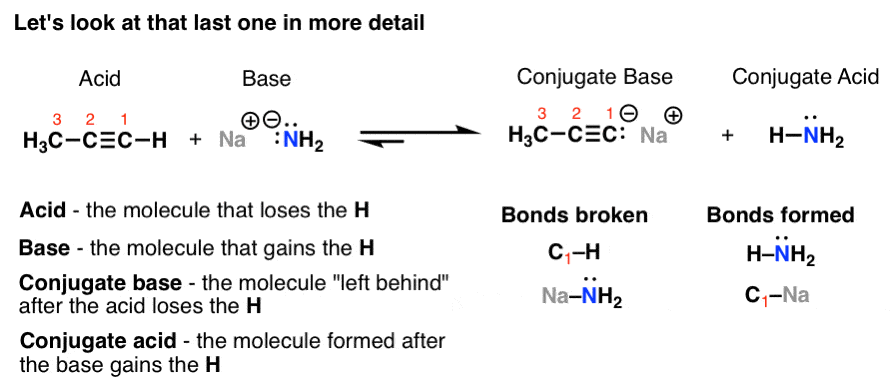
Introduction To Acid Base Reactions Master Organic Chemistry

How To Determine The Position Of Equilibrium For An Acid Base Reaction Chemistry Steps

Acid Base Reaction Definition Examples And Uses

How To Determine The Position Of Equilibrium For An Acid Base Reaction Chemistry Steps

Reversible And Irreversible Acid Base Reactions In Organic Chemistry

Acid Base Reactions In Organic Chemistry Master Organic Chemistry

How To Determine The Position Of Equilibrium For An Acid Base Reaction Chemistry Steps
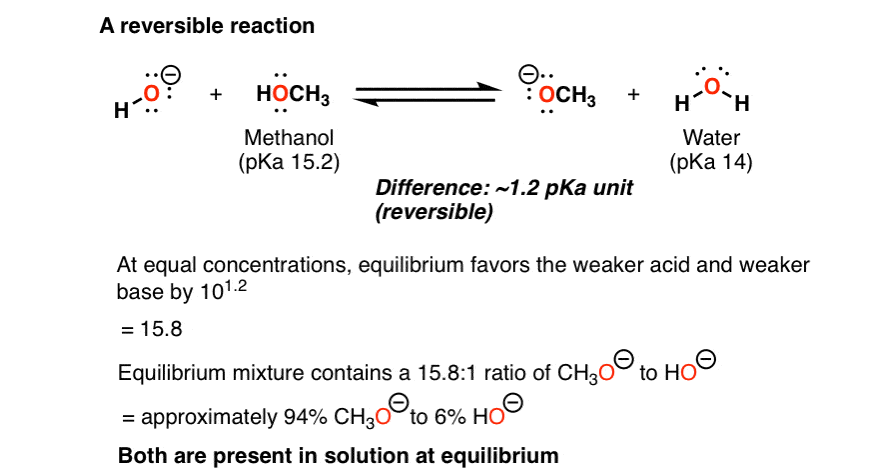
Reversible And Irreversible Acid Base Reactions In Organic Chemistry
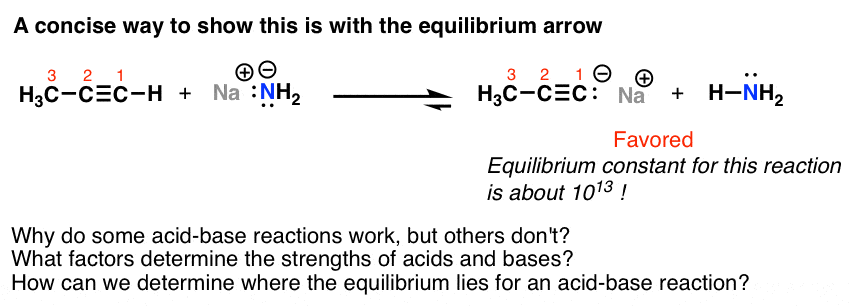
Introduction To Acid Base Reactions Master Organic Chemistry
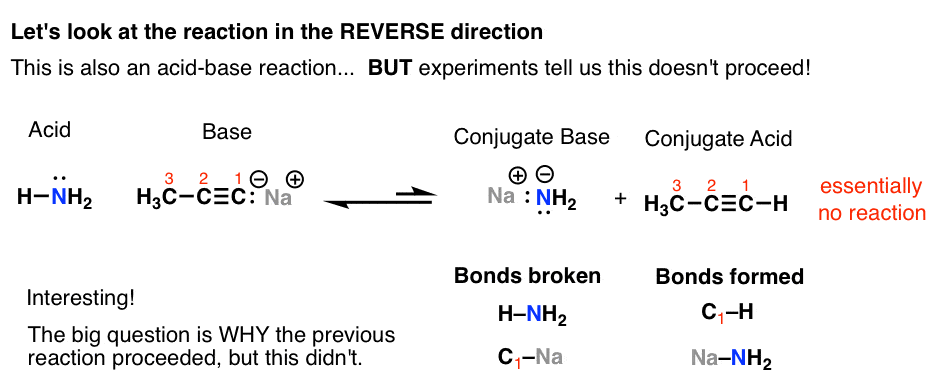
Introduction To Acid Base Reactions Master Organic Chemistry
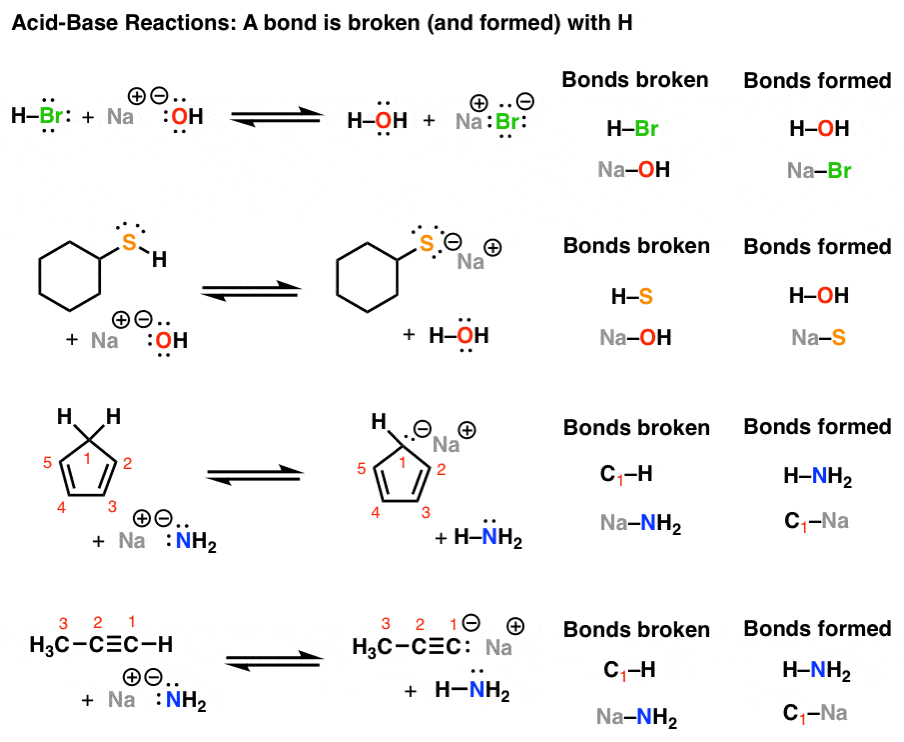
Introduction To Acid Base Reactions Master Organic Chemistry

14 1 Bronsted Lowry Acids And Bases Chemistry

Predicting Products Of Acid Base Reactions Youtube

Question Video Identifying Acids Bases And Salts In A Neutralization Reaction Nagwa
Comments
Post a Comment